One Health: Preventing and Solving Public Health Disasters

By Claudia Capos
Above. Joe Eisenberg stands in the Huron River to observe the interplay of natural ecosystems and human-built environments. Photo by Daryl Marscke
The emergence of the novel coronavirus SARS-CoV-2 in late 2019 and its rapid global spread have heightened the urgency of deploying better public health systems worldwide.
As we struggle to combat the current pandemic, we are faced with a question that public health experts have been posing for decades: Are we prepared for the next lethal disease outbreak?
The odds might not be in our favor.
Climate change, overpopulation, and food shortages are accelerating human encroachment into previously uninhabited areas, and putting people and livestock on a collision course with wildlife that harbor, and can transmit, novel diseases.

The coronavirus pandemic has been a wake-up call. It signals the need for greater coordination and cooperation among governments, public health officials, hospital systems, and community residents.
–Joseph Eisenberg, Professor of Epidemiology and Global Public Health
Veterinary, medical, and environmental surveillance systems are spotty and poorly interconnected, allowing deadly pathogens to spread stealthily and gain traction quickly in both humans and animals. Governments are reluctant or slow to report infectious disease outbreaks and often lack the will and resources to manage them.
“The coronavirus pandemic has been a wake-up call,” says Joseph Eisenberg, professor of Epidemiology and Global Public Health. “It signals the need for greater coordination and cooperation among governments, public health officials, hospital systems, and community residents.”
To achieve that coordination, many experts in the health sciences are promoting a One Health approach, a model centered on the idea that human health is intrinsically intertwined with the health of animals, plants, and our shared environments. One Health unites medical, veterinary, and environmental scientists in thinking more holistically about optimal health outcomes. And it engages the support of multiple sectors and levels of leadership, from local to global.
One Health is not a new concept. Some of its tenets can be traced to the ancient Greek physician Hippocrates (c. 460–c. 370 BC), who observed that disease was linked to environmental factors such as air and water as well as diet and living habits.
 “Over time, however, other concepts such as the biomedical model and the germ theory
shifted the public health focus away from socio-ecological considerations and toward
individual-level biomedical treatment,” Eisenberg says. The advent of vaccines and
antibiotics further weakened public health’s connection with the environment.
“Over time, however, other concepts such as the biomedical model and the germ theory
shifted the public health focus away from socio-ecological considerations and toward
individual-level biomedical treatment,” Eisenberg says. The advent of vaccines and
antibiotics further weakened public health’s connection with the environment.
“The One Health movement is an acknowledgement that we need to reorient ourselves and get back on track,” Eisenberg says. “Issues like climate change, lack of biodiversity, and degradation of the environment are impacting us and reminding us of our prior ways of thinking.”
Today, the One Health model is helping medical, veterinary, and environmental scientists address a variety of issues, including emerging infectious diseases, drug-resistant pathogens, noncommunicable diseases, and the impact of human behaviors on the planet’s ecosystem.
Rise in Animal-to-Human Spillover Events
Nearly three-quarters of new infectious diseases detected in recent decades have arisen through animal-to-human transmission, a process known as zoonosis.
 When a novel pathogen ― such as viruses, bacteria, protozoa, fungi, or prions―from
a wild or domestic animal infects a human and begins to replicate, it is a spillover
event. If the infected person spreads the pathogen to other people, and human-to-human
or even human-to-animal transmission continues, the result can be an explosive epidemic
such as those caused by the HIV and Ebola viruses.
When a novel pathogen ― such as viruses, bacteria, protozoa, fungi, or prions―from
a wild or domestic animal infects a human and begins to replicate, it is a spillover
event. If the infected person spreads the pathogen to other people, and human-to-human
or even human-to-animal transmission continues, the result can be an explosive epidemic
such as those caused by the HIV and Ebola viruses.
While the evolutionary history of the SARS-CoV-2 virus is uncertain, spillover events of other emerging infectious diseases are increasing.
“It’s beneficial for human medicine to understand the ecology and evolutionary biology of these zoonoses,” says Hayden Hedman, PhD ’19, an epidemiologist intelligence surveillance officer for the Centers for Disease Control and Prevention. “Knowing the trajectory of a disease in animal populations can help us predict and mitigate the cases and deaths in human populations.”
Several major drivers are contributing to spillover, according to Hedman.

Knowing the trajectory of a disease in animal populations can help us predict and mitigate the cases and deaths in human populations.
—Hayden Hedman, PhD ’19, Officer in the Epidemic Intelligence Service, Centers for Disease Control and Prevention
Changes in land use—road development, deforestation, urbanization, and agricultural intensification—directly impact the landscape, ecology, and social demography of remote areas. As people encroach on forested areas, they come into closer, more frequent contact with wildlife that may be reservoirs for novel diseases. In local markets where wild game is housed, slaughtered, and sold for food―alongside other wildlife and domestic animal species―there is heightened risk for cross-species spillover events to occur.
Other drivers of spillover have indirect or delayed effects. Biodiversity loss, for example, reduces the number of species that can act as a natural buffer for spillover. Climate change contributes to rising temperatures, which can accelerate the spread of some infectious diseases, such as dengue fever and Zika, to broader geographic areas. Human population growth also puts additional pressures on the environment that facilitate the emergence of zoonoses.
Emerging Disease and Improved Surveillance
Using an integrative One Health approach, epidemiologists can conduct both passive and active surveillance of animals and microorganisms to prevent the emergence of devastating pandemic outbreaks.
Passive surveillance involves clinically monitoring the number of cases of disease reported by physicians’ or veterinarians’ offices, hospital systems, and public health agencies. All too often these reports are incomplete, thereby providing an inaccurate assessment of the problem.
For clearer snapshots of community health, a growing number of countries worldwide use wastewater testing to monitor the spread of COVID-19 as well as other viral infections. Across the US, from New York to California, many communities have initiated COVID-19 sewershed surveillance projects to detect the presence of RNA from SARS-CoV-2 in their wastewater, a leading indicator of changes in the COVID-19 burden in a community.
 “We also need to broaden our concept of surveillance beyond tracking cases of disease
to tracking key environmental indicators,” says Hedman, “including surveillance of
pathogens in the environment and in animals.” Constructing a suite of early warning
indicators can help us to be more proactive and less reactive.
“We also need to broaden our concept of surveillance beyond tracking cases of disease
to tracking key environmental indicators,” says Hedman, “including surveillance of
pathogens in the environment and in animals.” Constructing a suite of early warning
indicators can help us to be more proactive and less reactive.
“It goes back to using a systems lens to look at this problem,” Hedman says. “We cannot take on an evolutionary arms race at the organismal level, because that’s futile. We must conduct comprehensive active surveillance.”
Proactive measures include:
- Monitoring geographic regions that have substantial overlapping of humans, wildlife, and livestock in low-hygiene settings
- Screening wet markets where vendors handle and sell wildlife, such as bats, wild cats, reptiles, and insects
- Conducting antibody testing to detect previous infections and unknown pathogens in animals before these emerge in humans
The transmission of viral and bacterial infections from animals to humans can happen in our own backyard. In the mid-1970s, northeastern US residents who were bitten by bacteria-carrying deer ticks while hiking or working outdoors developed a mysterious illness, later identified as Lyme disease. In the late 1990s, the West Nile virus, which is spread by infected mosquitos, began showing up in wild birds, putting humans and pets at risk.
Bovine tuberculosis, which usually causes disease in cattle, has been detected in northeastern Lower Michigan’s white-tailed deer population for many years, and on rare occasions humans have acquired spillover infections from activities related to deer hunting. In 2020, a multistate outbreak of human Salmonella Oranienberg infections was linked to pet turtles.

One Health emphasizes the importance of partnerships with environmental, animal, and human health practitioners.
—Laura Power, Director of the Office of Public Health Practice, Director of the Preventive Medicine Residency, and Clinical Assistant Professor of Epidemiology and Internal Medicine
“The complex problems we face in public health today need to be approached with cross-sector collaboration,” says Laura Power, clinical assistant professor of Epidemiology and Internal Medicine at the University of Michigan.
“One Health elevates this concept, emphasizing the importance of partnerships with environmental, animal, and human health practitioners,” Power says. “It can be challenging to bring together experts from across multiple sectors, but this collaborative approach has the potential to bring great long-lasting benefits.”
Proliferation of Drug-Resistant Pathogens
Antibiotics have helped to save the lives of countless humans, pets, and livestock.
However, the overuse of these drugs has resulted in an alarming proliferation of antibiotic-resistant pathogens. Drug-resistant strains of tuberculosis, salmonella, and MRSA threaten the health of millions of people worldwide.
“This is a crisis and has been for a long time,” says Betsy Foxman, professor of Epidemiology and director of the Center for Molecular and Clinical Epidemiology of Infectious Diseases. “There has been a push to get new antibiotics in the pipeline. But every time we have developed a new antibiotic, we have seen antibiotic resistance emerge.”

A One Health perspective makes it clear that to reduce antibiotic resistance takes a systems approach. We must work to reduce antibiotic use in agriculture, animal husbandry, and medicine.
—Betsy Foxman, Professor of Epidemiology and Director of the Center for Molecular and Clinical Epidemiology of Infectious Diseases
Bacteria can divide rapidly, as fast as every 15 to 20 minutes within the body. Each time bacteria divide, there is a chance of a mutation that can result in drug resistance. Further, bacteria can obtain antibiotic-resistance genes directly from other bacteria via a process called horizontal gene transfer, enabling antibiotic resistance to spread quickly.
“We’ve seen the exchange of multidrug-resistant super packages among different microbes circulating in hospital environments,” Foxman says. “This is very scary.”
Antibiotic-resistant organisms can be acquired via food. When antibiotics are fed to chickens, cows, fish, and pigs, antibiotic-resistant microbes can result and be passed on to humans and animals who consume this food. These antibiotic-resistant microbes are eventually excreted from the hosts and end up contaminating the water and soil. Using animal manure to fertilize vegetable gardens, pasturelands, and commercial crops also puts humans and animals at increased risk of ingesting drug-resistant organisms.
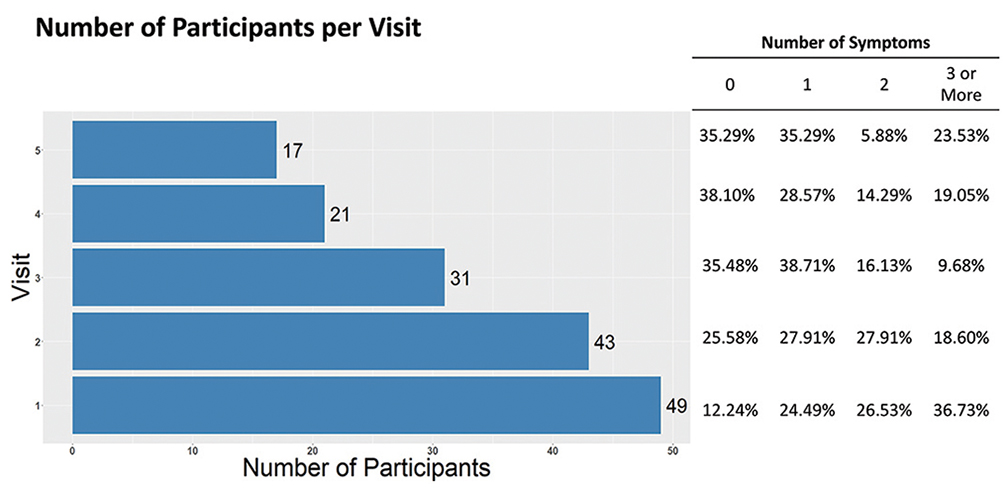
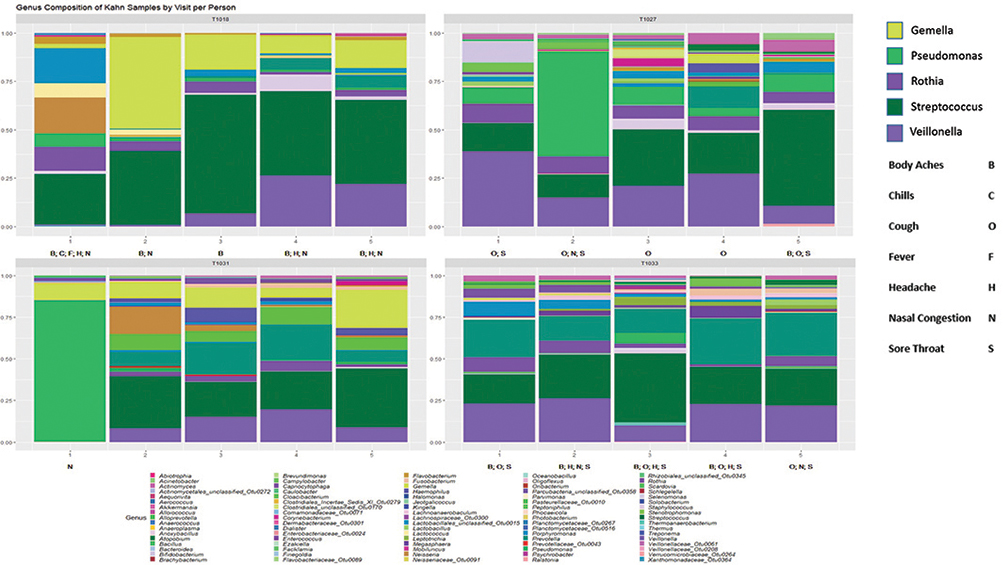
Foxman hypothesizes that microbes found in the nose and throat predict risk of acquiring respiratory infections. The completed study will also show how the microbiome changes by time, antibiotic treatment, and influenza-like symptoms.
Above are preliminary results from an ongoing study in a skilled nursing facility testing this hypothesis. Participants are visited every three days for up to five visits or until discharge. Symptoms changed with time—upper panel—as did the relative abundance of bacteria present—lower panel (data from four participants followed for five visits). Each column is from a separate visit, and visits are ordered by time.
The gut microbiome (a collection of trillions of symbiotic microbial cells) works to protect humans, as well as animals, against pathogens by keeping harmful organisms from entering the body and stimulating the innate and adaptive immune system to fend off intruders. It also produces antimicrobial products and signals, and helps to maintain the structure and functioning of the gastrointestinal tract.
 However, antibiotics disrupt this delicately balanced gut microbiome by killing off
the “good” microbes as well as the “bad” ones, thereby weakening the body’s natural
defenses against disease.
However, antibiotics disrupt this delicately balanced gut microbiome by killing off
the “good” microbes as well as the “bad” ones, thereby weakening the body’s natural
defenses against disease.
Sometimes “friendly” microbes turn against us. For example, symbiotic bacteria in the nose and throat might actually help influenza virus invade cells, and the influenza helps the bacteria to cause pneumonia or ear infections. Antibiotics also can cause Candida albicans, a fungus commonly found in the mouth, vagina, and gut, to change form and invade cells, resulting in infection (for example, yeast infections or thrush).
“A One Health perspective makes it clear that to reduce antibiotic resistance takes a systems approach,” Foxman says. “We must work to reduce antibiotic use in agriculture, animal husbandry, and medicine.”
Foxman applies this type of systems thinking to explore how the microbiome works with or against our body’s immune system to enhance or resist infection by bacteria, virus, and fungi. Ultimately, she would like to find out how to push a microbiome that enhances risk of infection toward one that prevents infection. A healthy microbiome means fewer infections and fewer antibiotic treatments―and makes it more likely that antibiotics will be effective when we really need them.

Above. O’Neill with colleagues, including students, at Detroit’s Villages at Parkside. With Zachary Rowe—executive director for Friends of Parkside, a community-based organization in Detroit, and founding member of the Detroit Community-Academic Urban Research Center—O’Neill leads research projects looking at heat impacts in several communities on Detroit’s East Side and South Eastside.
Pictured (l-r) are Todd Ziegler, Dominic Bednar, Marie O’Neill, Zachary Rowe, Cherrel Manley, Nathan Carpenter, Angelina Palacios, Edward Lo.
Good health is not equally distributed across the population. There is a lot of evidence that structural racism and other unfair practices can cause stress and worse health for people.
—Marie O’NeilL, Professor of Environmental Health Sciences and Epidemiology
Combating the Increased Incidence of Noncommunicable Diseases
The One Health model also provides a 360-degree perspective of the risk factors and socio-economic influences that contribute to the increasing incidence of noncommunicable diseases―such as heart disease, stroke, cancer, diabetes, and chronic lung disease―and inflict harm on the environment and ecosystem.
“Good health is not equally distributed across the population,” says Marie O’Neill, professor of Environmental Health Sciences and Epidemiology. “There is a lot of evidence that structural racism and other unfair practices can cause stress and worse health for people.”
Poverty is another risk factor for non-infectious diseases. “Many times, people with less means are living in more polluted areas and also have less power to move or to demand that the pollutants be removed,” O’Neill says.
She sees some worrisome trends that are driving the rise in chronic diseases. These include:
- Increased rates of overweight and obesity worldwide
- Longer, more severe allergy seasons
- Worsening air and water pollution
- Infectious diseases that predispose people to develop chronic disease

Current human diets are out of line with planetary, human, and animal well-being. Humans are simultaneously suffering from noncommunicable diseases due to overconsumption of unhealthy foods and undernutrition due to the absence of diverse, healthy foods.
–Nathalie Lambrecht, PhD ’21
Balancing immediate needs for transportation, manufacturing, and food production with long-term needs for a healthy climate and a sustainable environment is challenging.
“Current human diets are out of line with planetary, human, and animal well-being,” says Nathalie Lambrecht, PhD ’21. “Humans are simultaneously suffering from noncommunicable diseases due to overconsumption of unhealthy foods and undernutrition due to the absence of diverse, healthy foods.”
 At the Potsdam Institute for Climate Impact Research in Germany, she is exploring ways to help small-scale livestock holders and farmers
in developing countries produce food sustainably to feed their families.
At the Potsdam Institute for Climate Impact Research in Germany, she is exploring ways to help small-scale livestock holders and farmers
in developing countries produce food sustainably to feed their families.
This “closed-loop” farming system, which integrates livestock and vegetable farming, can provide low-income populations with better nutrition and greater food security while protecting the environment from the ravages of large-scale livestock and agricultural operations. Supporting small-holder families with veterinary services, sanitation infrastructure, and education on safe livestock management and nutrition also can reduce the risk of zoonotic disease exposure.
From a One Health perspective, high-income countries need to reduce their consumption of unsustainable amounts of red meat and dairy products, which is driving environmental damage associated with intensive livestock production, and shift toward plant-based diets with smaller amounts of animal-source foods, according to Lambrecht.
Fulfilling the Promise of a One Health Future
The multitude of problems confronting our world today will never disappear entirely.
However, the One Health model will enable current―and next-generation―research scientists, public-health experts, educators, and policy makers to recognize the interconnectedness of everything in our world.
“We are rediscovering the importance of contextualizing population health and clinical medicine within an ecosystem of interdependent social and ecological processes,” says Eisenberg. “This is a concept we have known for millennia. We now need to create systems that acknowledge the fact that human health is intricately intertwined with animal and ecological health.”
By pooling their knowledge and resources, the scientific community will be able to develop and implement multipronged measures to ensure better health for people, animals, the environment, and, ultimately, our planet.
Does Biodiversity Buffer?
Biodiversity can function as a natural disease buffer. When a greater number of species is available in an ecosystem, infection is more likely to be spread across different species. Some of those species are efficient at transmitting the pathogen, others are not. A recent University of Michigan study, for example, showed that, in native bee populations, areas with a greater diversity of bee species present had the lowest levels of three viral pathogens common to bees.
Disease ecologists are actively discussing the dilution effect hypothesis, a recent and well-known theory that would also predict the inverse—in systems with low species diversity, pathogen burden is higher among some species. This dilution effect remains a debated theory, in part, because we have many examples where increased biodiversity has neutral or negative effects on disease spread.
 Dilution has best been illustrated in vector-borne diseases where humans are accidental
or dead-end hosts. With Lyme disease, for example, the tick-borne bacterial pathogen
(Borrelia burgdorferi) does not depend on infecting humans to maintain transmission and persist but can
infect them occasionally and, biologically speaking, accidentally.
Dilution has best been illustrated in vector-borne diseases where humans are accidental
or dead-end hosts. With Lyme disease, for example, the tick-borne bacterial pathogen
(Borrelia burgdorferi) does not depend on infecting humans to maintain transmission and persist but can
infect them occasionally and, biologically speaking, accidentally.
When species diversity decreases, for example when predator populations are reduced, populations of prey who are Lyme reservoirs—mice, deer, and so on—likely will increase, increasing transmission in forest animals and subsequently increasing the risk that an infected tick will infect humans.
The dilution effect, after 15 years, is still a widely debated theory and not yet incorporated broadly into applied public health. Through a combination of field and modeling studies, One Health provides a framework where ecologists and public health researchers can work together to incorporate these concepts into practice.
—Hayden Hedman, PhD ’19
The One Health Model in Practice
Working across sectors and geographic regions to approach public health problems in unified, systematic ways sounds good. But it can be difficult to do. How well we integrate existing systems and develop new ones will determine our success.
One Health cannot be just modeling. It must become more than a high-level concept that informs research. One Health has to work on the ground. We’re starting to see how it can be implemented in local settings, but it depends on what disease you are focused on and what expertise your staff have. Stretching ourselves beyond zoonotic interactions to involve environmental health experts will also be important.
One Health cannot be just data collection. Local data has to be consistently and properly collected and fed up into high-level surveillance systems in a timely manner. Communication and training are vital here. Local researchers have to know what data is needed, how to collect it properly, and who needs it when.
Surveillance is the key to stopping spillover events from becoming pandemics, and surveillance happens when all levels of government collaborate. The CDC’s national forecasting plan will be a remarkable step toward this, but it will depend heavily on how well it can incorporate real-time data from counties and even smaller subregions around the country.
The complex problems we face in public health today need to be approached with cross-sector collaboration. One Health elevates and formalizes this concept, emphasizing the importance of partnerships with environmental, animal, and human health practitioners.
—Laura Power, Director of the Office of Public Health Practice, Director of the Preventive Medicine Residency, and Clinical Assistant Professor of Epidemiology and Internal Medicine
About the Author
Claudia Capos, a Michigan alumna, is an award-winning journalist, writer, and editor. Her feature articles on research, business, celebrities, and travel have appeared in national and international publications.
- Interested in public health? Learn more today.
- Read “How Museum Collections Can Enhance Public Health” [LINK once built]
- Support research and engaged learning at Michigan Public Health.
